Infectious Bursal Disease in Poultry
(Gumboro disease)
By
, PhD, Center for Food Animal Health, Department of Animal Sciences, College of Food, Agricultural, and Environmental Sciences, The Ohio State University
Last full review/revision Jul 2019
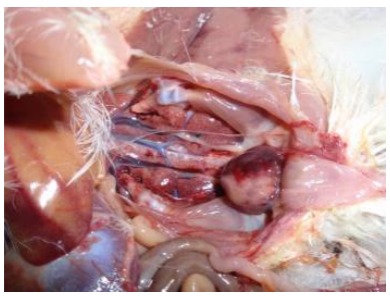
Infectious bursal disease (IBD) is seen in young domestic chickens worldwide and is caused by infectious bursal disease virus (IBDV). Signs can include depression, watery diarrhea, ruffled feathers, and dehydration. Morbidity is high and mortality is usually low, but some very virulent strains are capable of causing 60% or higher mortality. Macroscopic and microscopic lesions in the cloacal bursa and molecular identification of the viral genome are used to diagnosis the disease. Vaccination to induce maternal immunity in young chicks is initially used to control the disease. Vectored and live-attenuated vaccines can be used to induce active immunity in chicks as the maternal antibodies wane.
Infectious bursal disease (IBD) is seen in young domestic chickens worldwide and is caused by infectious bursal disease virus (IBDV). Symptoms of the clinical disease can include depression, watery diarrhea, ruffled feathers, and dehydration. Depending on the IBDV strain and presence of maternal immunity, the disease can also present as a clinical or subclinical disease in young chicks. For both clinical and subclinical forms of the disease, all pathogenic IBDVs cause lesions in the bursa of Fabricious. The cloacal bursa can become enlarged, with a yellowish colored transudate on the surface. Hemorrhages on the serosal and mucosal services are sometimes observed. Atrophy of the bursa, which includes the loss of B-lymphocytes, occurs approximately 7-10 days after infection. Immunosuppression is directly related to this loss of B-lymphocytes, but immunosuppression and related secondary infections are typically seen in birds that recover from the disease. Severity of the immunosuppression depends on the virulence of the infecting virus and age of the host.
Etiology and Transmission of Infectious Bursal Disease
Infectious bursal disease is caused by a birnavirus (infectious bursal disease virus; IBDV) that is most readily isolated from the bursa of Fabricius but may be isolated from other organs. It is shed in the feces and transferred from house to house by fomites. It is very stable and difficult to eradicate from premises.
Two serotypes of IBDV have been identified. The serotype 1 viruses cause disease in chickens and, within them, antigenic variation can exist between strains. Antigenic drift is largely responsible for this antigenic variation, but antigenic differences can also occur through genome homologous recombination. Serotype 2 strains of the virus infect chickens and turkeys but have not caused clinical disease or immunosuppression in these hosts. IBDVs have been identified in other avian species, including penguins, and antibodies to IBDV have been seen in several wild avian species. The contribution of IBDV to disease in these wild birds is unknown.
Clinical Findings of Infectious Bursal Disease
Infectious bursal disease is highly contagious; results of infection depend on age and breed of chicken and virulence of the virus. Infections may be subclinical or clinical. Infections before 3 weeks of age are usually subclinical. Chickens are most susceptible to clinical disease at 3–6 weeks of age when immature B cells populate the bursa and maternal immunity has waned, but severe infections have occurred in Leghorn chickens up to 18 weeks of age.
Early subclinical infections are the most important form of the disease because of economic losses. They cause severe, long-lasting immunosuppression due to destruction of immature lymphocytes in the bursa of Fabricius, thymus, and spleen. The humoral (B cell) immune response is most severely affected; the cell-mediated (T cell) immune response is affected to a lesser extent. Chickens immunosuppressed by early IBDV infections do not respond well to vaccination and are predisposed to infections with normally nonpathogenic viruses and bacteria. Common diseases are usually exacerbated by IBDV infections. Some strains of IBDV can cause subclinical infections in older birds (3–6 weeks old), which leads to losses from poor feed efficiency and longer times to market. In these cases, the immunosuppression is usually transient, and convalescent birds may recover most or all of their humoral immune function. However, secondary infections that occur during the transient immunosuppression can cause significant economic losses.
In clinical infections, onset of the disease occurs after an incubation of 3–4 days. Chickens may exhibit severe prostration, incoordination, watery diarrhea, soiled vent feathers, vent picking, and inflammation of the cloaca. Flock morbidity is typically 100%, and mortality can range from 5% to greater than 60% depending on the strain of virus and breed of chicken. Mortality is typically higher in layer breeds compared with broiler chickens. Recovery occurs in < 1 week, and broiler weight gain is delayed by 3–5 days. The presence of maternal antibody will modify the clinical course of the disease.
Virulence of field strains of the virus varies considerably. Viruses that range from naturally attenuated to very virulent (vv) have been observed. The vvIBDV strains that can cause high mortality (>20%) were first detected in Europe. They spread throughout the Middle East, Asia, and Africa, were detected in South and Central America in 1999, and in the USA in 2009. At necropsy, the lesions seen will depend on the strain of IBDV. For strains that cause a clinical disease, the cloacal bursa is swollen, edematous, yellowish, and occasionally hemorrhagic, especially in birds that died of the disease. Strains of vvIBDV cause similar cloacal bursa lesions, and congestion and hemorrhage of the pectoral and leg muscles can also occur. Some IBDV strains can cause atrophy of the cloacal bursa without the appearance of gross lesions in that organ. Chickens that have recovered from IBDV infections have small, atrophied, cloacal bursas due to the destruction and lack of regeneration of the bursal follicles.
Diagnosis of Infectious Bursal Disease
-
Diagnosis can be accomplished by clinical evaluation of the cloacal bursa for macroscopic and microscopic lesions followed by molecular detection of the viral VP2 gene using RT-PCR
-
Sequence analysis of the VP2 gene is used to identify the IBDV genotype
-
Virus isolation in chicken embryos or chicken embryo fibroblast cell cultures is possible but often not necessary
Initial diagnosis of infectious bursal disease is accomplished by the observation of gross lesions in the cloacal bursa. This is followed by microscopic analysis of the bursa for lymphocyte depletion in the follicles. Molecular diagnostic assays are most often used to identify IBDV in diagnostic samples. The reverse-transcriptase-PCR assay is used to identify the viral genome in bursa tissue. Sequence alignments and phylogenetic analysis of the VP2 coding region has been used to further characterize the viruses into genogroups. Samples for molecular diagnostic testing are typically collected after maternal antibodies have waned. IBDV may be isolated in 8- to 11-day-old, antibody-free chicken embryos with inocula from birds in the early stages of disease. The chorioallantoic membrane is more sensitive to inoculation than is the allantoic sac. Some strains of IBDV may also be isolated in cell cultures that include chicken embryo fibroblasts, cells from the cloacal bursa, and established avian and mammalian cell lines. Cell culture–adapted strains of IBDV produce a cytopathic effect and may be used for quantitative titration of the virus and virus-neutralization assays.
Serology can be used to detect the presence of antibodies to IBDV in convalescent chicks. Commercially available ELISA kits are most often used to quantitate IBDV antibodies. The presence of IBDV antibodies in chicks is not always an indication of infection because most young chicks have maternal antibodies.
Control and Treatment of Infectious Bursal Disease
There is no treatment. Rigorous disinfection of contaminated farms after depopulation has achieved limited success. Live vaccines of chicken embryo or cell-culture origin and of varying low pathogenicity can be administered by eye drop, drinking water, or SC routes at 1–21 days of age. Replication of these vaccines and thus the immune response can be altered by maternal antibody, although the more virulent vaccine strains can override higher levels of maternal antibody. Vectored vaccines that express the IBDV VP2 protein in herpesvirus of turkeys (HVT) can be used in ovo or at hatch. These HVT-IBD vaccines are not affected by maternal antibodies. Vaccines that use live-attenuated viruses bound to antibodies (immune-complex vaccines) are also available for in ovo or at hatch administration.
High levels of maternal antibody during early brooding of chicks in broiler flocks (and in some commercial layer operations) can minimize early infection, subsequent immunosuppression, or both. Breeder flocks should be vaccinated one or more times during the growing period, first with a live vaccine and again just before egg production with an oil-adjuvanted, inactivated vaccine. Inactivated vaccines of chicken embryo, bursa, or cell-culture origin are available. The latter vaccines induce higher, more uniform, and more persistent levels of antibody than do live vaccines. The immune status of breeder flocks should be monitored periodically with a quantitative serologic test such as virus neutralization or ELISA. If antibody levels decrease, hens should be revaccinated to maintain adequate immunity in the progeny.
The goal of any vaccination program for IBD should be to use vaccines that most closely match the antigenic profile of the field viruses. Diagnostic testing for the genomic sequences of field strains can be used to select the most appropriate vaccination program.
Key Points
-
Infectious bursal disease, caused by infectious bursal disease virus, is a disease of young chickens. The virus infects immature B-lymphocytes and causes an immune suppression that leads to secondary infections in convalescent birds.
-
The virus is found worldwide, and diagnosis is through clinical evaluation of the cloacal bursa and molecular identification of the viral genome.
-
Control of IBD is accomplished using vaccination of breeder flocks to induce maternal immunity in young chicks. Antigenic drift requires the use of vaccines that closely match the antigenic structure of the infecting virus. Vaccination in ovo or young chicks using vectored or live-attenuated vaccines can help boost protection as maternal antibodies wane.