My Store
PHARMAGAL-BIO Pigeon Healthcare. RP Vaccine - Herpesvirus Vaccine - Adenovirus Vaccine.
PHARMAGAL-BIO Pigeon Healthcare. RP Vaccine - Herpesvirus Vaccine - Adenovirus Vaccine.
Couldn't load pickup availability
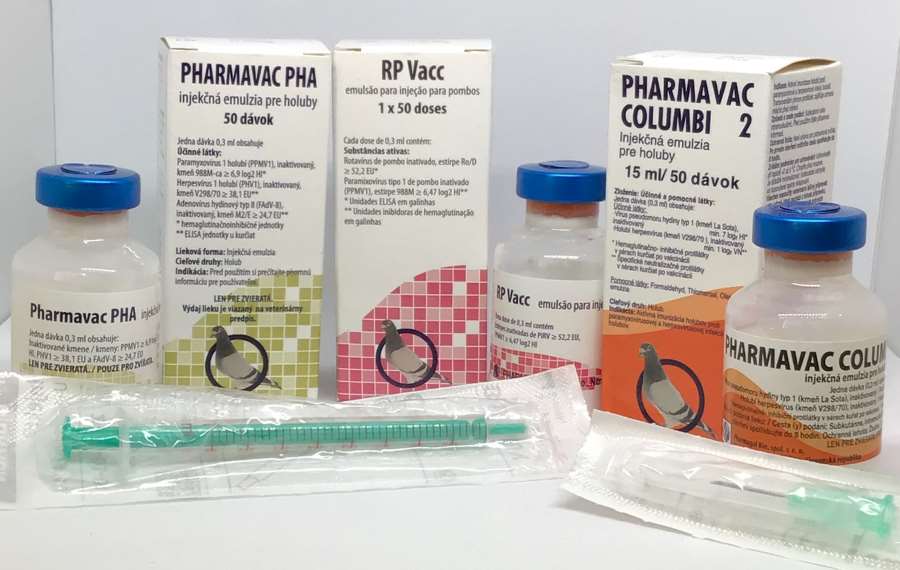
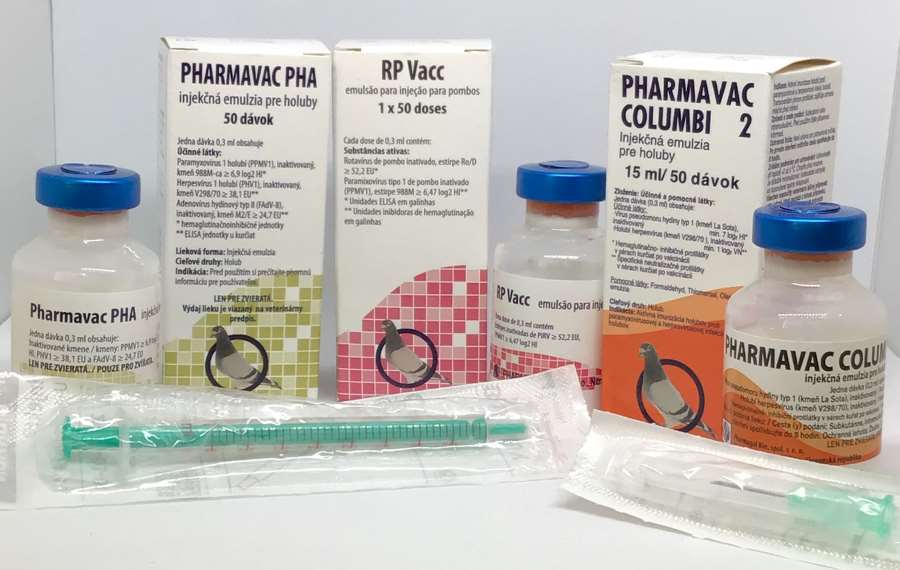
RP Vacc Emulsion for Injection for Pigeons
1. VETERINARY MEDICINAL PRODUCT NAME
- RP Vacc emulsion for injection for pigeons (BE, CZ, DE, NL, PL, PT, RO, SK)
- RP Vacc vakcina A.U.V. (HU)
2. ACTIVE SUBSTANCE(S) AND OTHER INGREDIENT(S)
Each 0.3 ml dose contains:
-
Active substances:
- Inactivated Pigeon Rotavirus, strain Ro/D: ≥ 52.2 EU*
- Inactivated Pigeon Paramyxovirus type 1 (PPMV1), strain 988M: ≥ 6.47 log2 HI**
-
Adjuvants:
- Paraffin oil: 156.9 mg
- Sorbitan oleate: 15.8 mg
- Polysorbate 80: 5.7 mg
-
Excipient:
- Thiomersal: max. 0.036 mg
*ELISA units in chicken
**Haemagglutination inhibition in chicken
- Thiomersal: max. 0.036 mg
3. INDICATIONS
For active immunization of pigeons from 4 weeks of age:
- Reduce clinical signs and virus shedding from pigeon rotavirus.
- Reduce mortality and severity of clinical signs caused by paramyxovirus type 1 (PMV1).
Immunity Onset: 2 weeks post-primary vaccination
Immunity Duration: 8 months (PiRV), 9 months (PMV1)
4. CONTRAINDICATIONS
None.
5. ADVERSE REACTIONS
Common: Mild apathy and site pain after injection (lasting ≤1 day).
Report any side effects to your veterinarian.
6. TARGET SPECIES
Pigeons.
7. DOSAGE AND ADMINISTRATION
- Dosage: 0.3 ml intramuscularly in the femoral muscle.
-
Primary Vaccination:
- First dose: From 4 weeks of age.
- Second dose: 3 weeks later.
- Revaccination: One dose every year; in high-risk flocks, every 8-9 months.
8. ADMINISTRATION ADVICE
- Administer at an acute angle.
- Shake well before use.
- Warm to room temperature prior to administration.
9. WITHDRAWAL PERIOD
Zero days.
10. STORAGE PRECAUTIONS
- Store in a refrigerator (2°C – 8°C), protect from frost and light.
- Shelf life after opening: 8 hours.
11. SPECIAL WARNINGS
- Vaccinate healthy animals only.
- Assess timing based on disease prevalence.
For the person administering the product: Accidental self-injection may cause severe pain; seek immediate medical advice.
12. DISPOSAL
Consult your veterinarian or pharmacist for proper disposal of unused product.
13. LAST APPROVAL DATE
[Insert Date]
14. PACKAGE SIZE
Cardboard box containing 1 vial of 50 doses.
_____________________________________________________________________________________________
PHARMAVAC COLUMBI 2 emulsion for injection for Herpesvirus and Paramyxovirus Type 1 for pigeons:
1. MARKETING AUTHORISATION HOLDER AND MANUFACTURING AUTHORISATION HOLDER
- Holder: Pharmagal Bio spol. s r.o.
- Address: Murgašova 5, 949 01 Nitra, Slovak Republic
2. PRODUCT NAME
PHARMAVAC COLUMBI 2 Emulsion for Injection for Pigeons
3. ACTIVE SUBSTANCES AND INGREDIENTS
Each dose (0.3 ml) contains:
-
Active Substances:
- Inactivated Paramyxovirus Type 1 (strain La Sota): min. 7 log2 HI*
- Inactivated Pigeon Herpesvirus (strain V298/70): min. 1 log2 VN**
- HI: Haemagglutination-inhibition antibodies in serum from vaccinated chickens
** VN: Specific virus neutralisation antibodies in serum from vaccinated chickens
- Excipients: Formaldehyde, Thiomersal, Oily emulsion
4. INDICATIONS
For active immunisation of pigeons against paramyxovirus and herpesvirus infections.
- Onset of Immunity: 14 days post last injection
- Duration of Immunity: 1 year
5. CONTRAINDICATIONS
Do not vaccinate animals that are ill or suspected of being ill.
6. ADVERSE REACTIONS
No known adverse reactions. If any serious effects or unlisted effects occur, consult a veterinary surgeon.
7. TARGET SPECIES
Pigeons.
8. DOSAGE AND ADMINISTRATION
Administer subcutaneously in the neck area or intramuscularly in the femoral or breast muscle.
- Dosage for All Ages: 0.3 ml
Vaccination of young pigeons induces antibody production against viral antigens within 10-14 days post first administration. A booster dose should be given 3 to 5 weeks after the initial injection.
- Revaccination: Annually; for adult pigeons in high-risk breeding, vaccination is recommended twice a year, within a maximum interval of 6 months.
9. ADMINISTRATION ADVICE
Vaccinate pigeons prior to the egg-laying period. The vaccine promotes antibody production, providing young birds with protection via transovarian transfer.
10. WITHDRAWAL PERIOD
None.
11. STORAGE PRECAUTIONS
- Keep out of reach of children.
- Store in a refrigerator at 2-8°C.
- After opening, use within 8-10 hours.
- Protect from light and store in a dry place.
- Do not use after the expiry date on the label.
12. WARNINGS
- No data on the safety and efficacy of this vaccine with other veterinary medicinal products; assess each case individually.
- Do not mix with other veterinary medicinal products without compatibility studies.
- User Warning: Contains mineral oil. Accidental injection may cause severe pain and swelling. Seek medical attention immediately if injected.
- Physician Warning: Accidental injection can lead to significant swelling and potential loss of digits; prompt surgical intervention may be necessary.
13. DISPOSAL OF UNUSED PRODUCT
Consult your veterinary surgeon for proper disposal of unused medicines. Dispose of in accordance with local regulations.
14. LAST APPROVAL DATE
5.5.2010
15. ADDITIONAL INFORMATION
- Available only on veterinary prescription.
- For animal treatment only.
-
Package Sizes: 15 ml (50 doses), 30 ml (100 doses).
(Note: Not all pack sizes may be available.) _______________________________________________________________________________________PHARMAVAC PHA, PMV 1- Herpesvirus - Adenovirus Vaccine.PACKAGE LEAFLET: PHARMAVAC PHA Emulsion for Injection for Pigeons
1. MARKETING AUTHORISATION HOLDER AND MANUFACTURING AUTHORISATION HOLDER
- Holder: PHARMAGAL-BIO spol. s r.o.
- Address: Murgašova 5, 94901 Nitra, Slovak Republic
2. PRODUCT NAME
PHARMAVAC PHA Emulsion for Injection for Pigeons
3. ACTIVE SUBSTANCES AND INGREDIENTS
Each vaccine dose (0.3 ml) contains:
-
Active Substances:
- Inactivated Pigeon Paramyxovirus Type 1 (PPMV1), strain 988M: ≥ 6.9 log2 HIU*
- Inactivated Pigeon Herpesvirus 1 (PHV1), strain V298/70: ≥ 38.1 EU**
- Inactivated Fowl Adenovirus Type 8 (FAdV-8), strain M2/E: ≥ 24.7 EU**
- HIU: Haemagglutination inhibition units in chickens
** EU: ELISA units in chickens
-
Adjuvants:
- Paraffin oil: 156.9 mg
- Sorbitan oleate: 15.8 mg
- Polysorbate 80: 5.7 mg
-
Excipients:
- Formaldehyde: max. 0.060 mg
- Thiomersal: max. 0.036 mg
Appearance: White emulsion with easily shakeable sediment.
4. INDICATIONS
For active immunization of pigeons from 4 weeks of age:
-
To reduce mortality and the frequency and severity of clinical signs caused by Paramyxovirus Type 1 (PMV1).
-
To mitigate clinical signs, gross lesions, and virus shedding due to Pigeon Herpesvirus (PHV1).
-
To lessen the severity of clinical signs and lesions caused by Fowl Adenovirus Types 7/E, 2/D, 3/D, and 4/C (subgroup I).
-
Onset of Immunity: 3 weeks post-vaccination
-
Duration of Immunity:
- 12 months for PMV1
- 5 months for PHV1 and AdV (demonstrated by cell-mediated immunity and serological data)
5. CONTRAINDICATIONS
None.
6. ADVERSE REACTIONS
Common: Slight swelling (up to 1.0 cm) at the injection site, resolving within 9 days.
Frequency of adverse reactions:
- Very common: >10%
- Common: >1% but <10%
- Uncommon: >0.1% but <1%
- Rare: >0.01% but <0.1%
- Very rare: <0.01%
Report any side effects to your veterinary surgeon.
7. TARGET SPECIES
Pigeons.
8. DOSAGE AND ADMINISTRATION
- Dosage: 0.3 ml
- Administer subcutaneously in the dorsal neck region, away from the head, starting at 4 weeks of age.
9. ADMINISTRATION ADVICE
- Shake well before use.
- Allow vaccine to reach room temperature before administration.
- Use sterile syringes and needles.
- Ensure proper dosing with graduated syringes.
10. WITHDRAWAL PERIOD
Zero days.
11. STORAGE PRECAUTIONS
- Keep out of reach of children.
- Store in a refrigerator (2°C – 8°C).
- Protect from frost and light.
- Do not use after the expiry date on the label.
- Shelf life after opening: 8 hours.
12. WARNINGS
- Vaccinate only healthy animals.
- Timing of vaccination should be based on a risk-benefit assessment by a veterinarian, considering disease prevalence and risk periods.
- Avoid injecting into sites previously vaccinated subcutaneously.
User Safety:
- Contains mineral oil; accidental injection may cause severe pain and swelling. Seek medical attention immediately if injected.
- To the physician: Immediate expert surgical intervention may be necessary for accidental injections.
13. DISPOSAL OF UNUSED PRODUCT
Consult your veterinary surgeon or pharmacist for proper disposal methods to protect the environment.
14. LAST APPROVAL DATE
09/2018
15. ADDITIONAL INFORMATION
- Package Size: Box containing 1 vial of 50 doses.
Racing Pigeon Laboratory Testing . com
RP Diagnostic Laboratory .com
Share