My Store
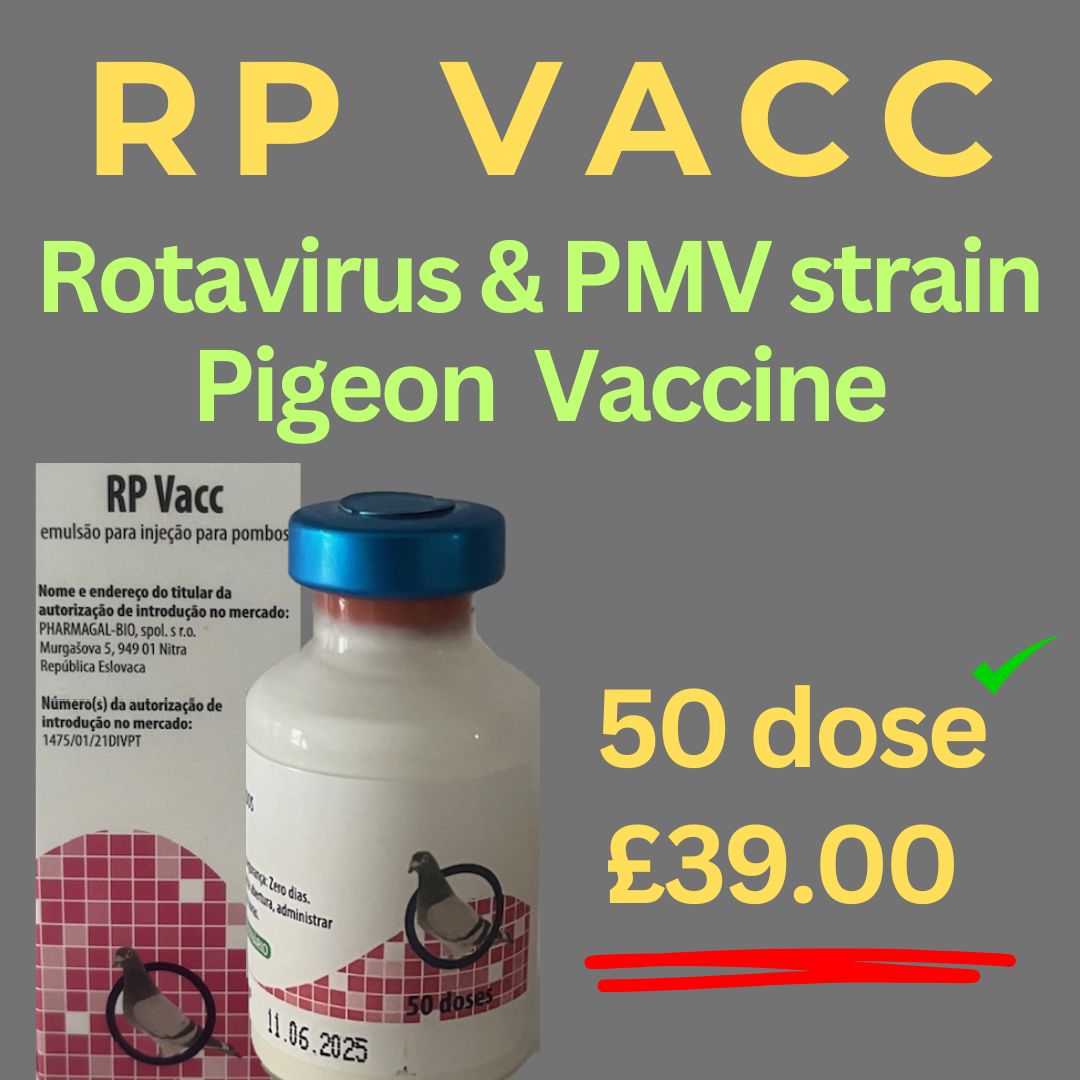
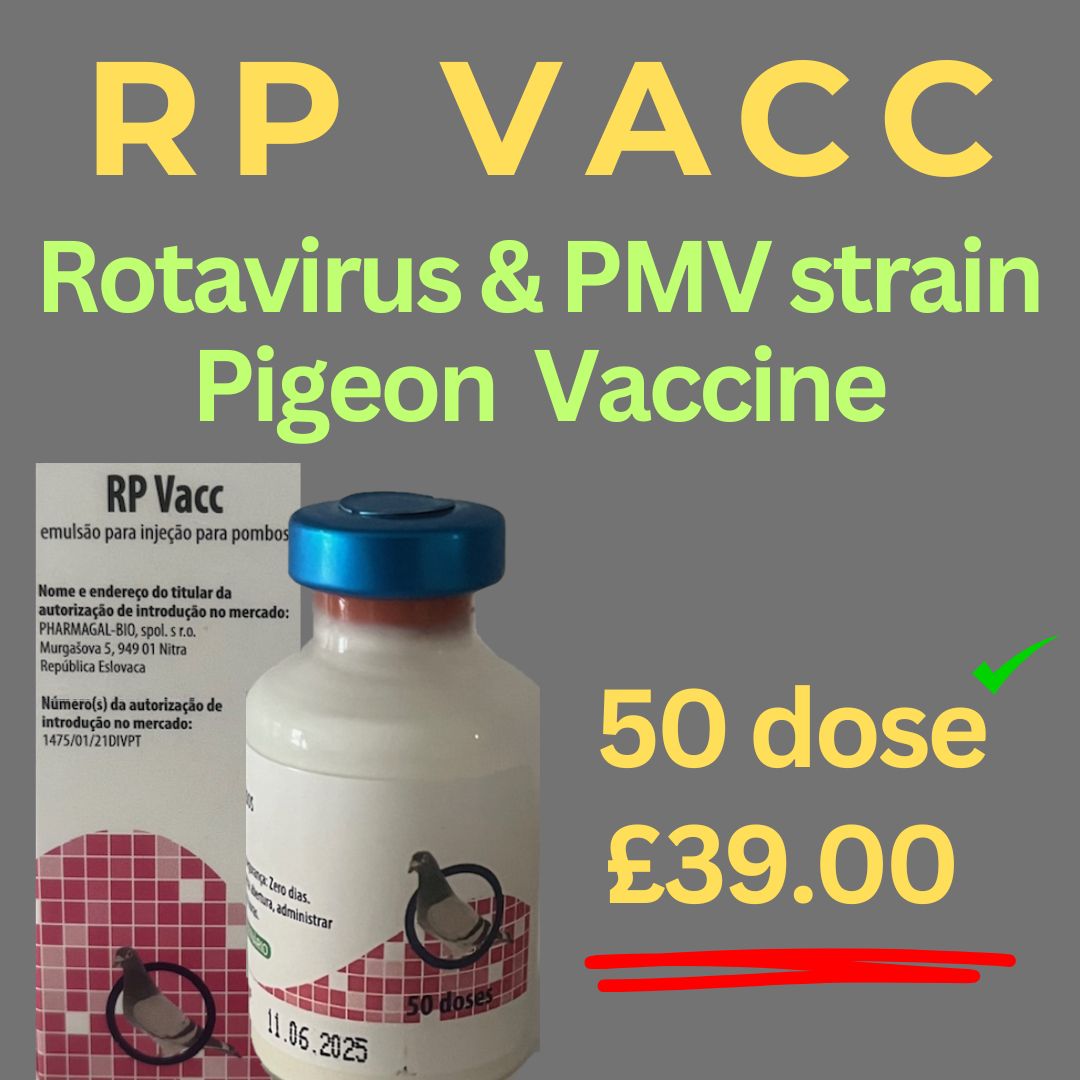
RP-Rotavirus & Ro-DParamyxovirus Type 1 (PPMV1) 2 in vaccine 50 dose
RP-Rotavirus & Ro-DParamyxovirus Type 1 (PPMV1) 2 in vaccine 50 dose
Couldn't load pickup availability
RP Vaccine & PPMV 1 emulsion for injection for pigeons
Active substances:
Inactivated pigeon rotavirus, Ro/D strain
Inactivated pigeon paramyxovirus type 1 (PPMV1), strain 988M ≥ 6.47 log2 H|**
* ELISA units in chickens; ** Haemagglutination inhibiting units in chickens
Adjuvants: Paraffin oil....156.9 mg; Sorbitan oleate...15.8 mg; Polysorbate 80...5,7 mg
Excipients: Tiomersal...max. 0.036 mg
White emulsion with easily dispersible sediments.
INDICATION (INDICATIONS): For active immunisation of pigeons from 4ª week of life:
-To reduce the frequency and severity of clinical signs, pathological lesions and spread of the virus caused by group A pigeon rotavirus, G18P|17 genotype (PIRV),
- To reduce mortality and the frequency and severity of clinical symptoms caused by paramyxovirus type 1 (PMV1).
Beginning of immunity: 2 weeks after the completion of the basic vaccination schedule
Duration of immunity: 8 months (PiRV) / 9 months (PMV1) after the completion of the basic vaccination schedule (demonstrated by virulent evidence)
In field studies, antibody levels comparable to those demonstrated by virulent evidence were found, even one year after the last injection.
9. INSTRUCTIONS FOR CORRECT USE: When administering the vaccine, it is recommended that the inclination of the needle in relation to the muscle be at an acute angle, not perpendicular to the site of administration.
Shake before use and occasionally during use. Let the vaccine reach room temperature before use. Apply under normal aseptic conditions, using sterile syringes and needles. Use properly graded syringes that allow the administration of an accurate dose of 0.3 ml vaccine.
10. SAFETY INTERVAL(S): Zero days.
11. SPECIAL PRECAUTIONS FOR CONSERVATION
• Keep out of the sight and reach of children.
Store in the refrigerator (2 °( - 8 °C). Avoid freezing. Protect from light. Do not use this veterinary medicinal product after the expiry date stated on the label.
Shelf life after first opening the container: 8 hours
12. SPECIAL WARNING(S)
Special warnings for each target species: Vaccinate only healthy animals.
The time of vaccination/revaccination should be based on a risk-benefit assessment by the responsible veterinarian, taking into account the prevalence of specific diseases in reproduction and the periods of greatest risk of transmission of the disease (i.e., start of the flight season, exhibition season and/or breeding season).
In the field study, the presence of antibodies of maternal origin for PiRV did not show a negative effect on the development of a post-vaccination antibody response.
Special precautions for use in animals: Not applicable.
Special precautions to be taken by the person administering the veterinary medicinal product to animals: In case of accidental self-injection, go to a doctor immediately and show him the package leaflet or the label.
Warning to the user: This veterinary medicinal product contains mineral oil. Accidental injection or self-injection may cause
Severe pain and swelling, particularly if injected into a joint or finger, which may, in rare cases, result in the loss of the affected finger, if immediate medical care is not provided. In case of accidental injection, and even if the amount injected is minimal, immediately consult a doctor and show the package leaflet of the medicine.
This veterinary medicinal product contains mineral oil. Accidental injection, even in a small amount, can cause intense swelling that can result, for example, in ischaemic necrosis and, if the affected area is a finger, in the loss of it. It is necessary to provide IMMEDIATE surgical care, and it may be necessary to make an early incision and irrigation of the injected area, especially if it involves the soft tissues or the tendon of a finger.Egg laying: The safety of the veterinary medicine has not been determined during egg laying. Administer only in accordance with the benefit/risk assessment carried out by the responsible veterinarian.
Drug interactions and other forms of interaction: There is no information available on the safety and efficacy of this vaccine when used with any other veterinary medicinal product. The decision to administer this vaccine before or after the administration of another veterinary medicinal product should be made on a case-by-case basis.
Overdose (symptoms, emergency procedures, antidotes): Not applicable.
Main incompatibilities: In the absence of compatibility studies, this veterinary medicinal product should not be mixed with others.
13. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED MEDICINAL PRODUCT OR WASTE MATERIALS, IF ANY
Ask your veterinarian or pharmacist how to dispose of veterinary medicines that are no longer necessary. These measures contribute to the protection of the environment.
14. DATE OF LAST APPROVAL OF THE PACKAGE LEAFLET
12/2021
Ro/D strain Rotavirus is a specific strain of rotavirus that affects pigeons, leading to a disease known as "pigeon rotavirus infection." This virus is part of the larger family of rotaviruses, which are RNA viruses known to cause gastrointestinal infections in various animals, including birds and mammals.
Characteristics of Ro/D Strain Rotavirus in Pigeons
-
Disease Presentation:
- Symptoms: In pigeons, infection with the Ro/D strain of rotavirus typically results in severe gastrointestinal symptoms, such as diarrhea, dehydration, and in some cases, vomiting. Affected pigeons may also exhibit lethargy, reduced appetite, and weight loss.
- Severity: The Ro/D strain is known to cause particularly severe outbreaks, leading to high mortality rates, especially in young or immunocompromised birds. Adult pigeons can also be affected, though they may sometimes show milder symptoms.
-
Transmission:
- The virus spreads primarily through the fecal-oral route. This means that pigeons contract the virus by ingesting contaminated food, water, or through direct contact with infected birds or surfaces.
- The virus is highly contagious, making it a significant concern in environments where pigeons are kept in close quarters, such as lofts or aviaries.
-
Diagnosis:
- Diagnosis of the Ro/D strain rotavirus in pigeons is typically confirmed through laboratory testing, including PCR (Polymerase Chain Reaction) assays that detect the viral RNA.
- Histopathological examination of the intestines and other affected organs can also be used to identify the characteristic lesions caused by the virus.
-
Prevention and Control:
- Hygiene: Strict hygiene practices, including regular cleaning and disinfection of lofts, water, and feeding equipment, are crucial in preventing the spread of the virus.
- Quarantine: New birds should be quarantined before being introduced to an existing flock to prevent the introduction of the virus.
- Vaccination: While there is ongoing research into vaccines, as of now, the RP Vaccine is available specifically for the Ro/D strain in pigeons. However, in some regions, autogenous vaccines are being developed and used to manage outbreaks.
-
Treatment:
- There is no specific antiviral treatment for rotavirus in pigeons. Management typically focuses on supportive care, such as providing fluids to combat dehydration and ensuring adequate nutrition. Probiotics and electrolyte solutions might also be used to support recovery.
- In some cases, secondary bacterial infections can occur due to the compromised gut, and antibiotics might be prescribed to manage these infections.
The Ro/D strain of rotavirus in pigeons represents a significant health threat to pigeon populations, particularly in breeding or racing settings, where the close proximity of birds can facilitate rapid spread.
Share